Happy Independence Day!
This 4th of July our nation prepares to celebrate its 243rd birthday. While we here at Microtrace join in the celebrations and applaud the grand fireworks displays, we continue to marvel at the microscopic. So, as you enjoy the astonishing explosions and beautiful colors erupting in the sky above, give some thought to the chemistry that makes them possible.
The brilliant colors at which we marvel result from the combustion of various metallic salts. These include strontium carbonate (red), copper(II) chloride (blue), barium chloride (green), and sodium nitrate (yellow).

From left to right: Strontium carbonate, copper(II) chloride, barium chloride, and sodium nitrate.
Like gunshot residue (GSR), fireworks and other low explosives are capable of releasing enough energy to produce molten reaction products- pyrotechnic reaction residue (PRR).

Sequential images of the combustion of a Roman candle, in which the fine cloud of pyrotechnic reaction residue (PRR) can be observed.
The combustion products cool quickly while still airborne and solidify into spheroidal shapes. The resulting residue is a mixture consisting of both combustion products and unconsumed material. In fact, the production, sampling, and analysis of PRR are typically similar to that of GSR. Analyses involve the use of SEM analysis combined with energy dispersive X-ray spectroscopy (SEM/EDS). In many cases, advanced microchemical testing can be used to determine additional information about the explosives residue.
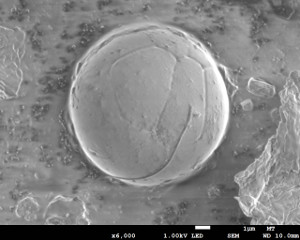
A low explosive residue particle.
From all of us here at Microtrace: We hope you have a relaxing, enjoyable, and safe 4th of July.
How May We Help You?
Contact usto discuss your project in more detail.








